Review of the NICE-SUGAR Trial
Intensive versus Conventional Glucose Control in Critically Ill Patients
N Engl J Med 2009;360:1283-97.
Background Patients with diabetes have higher short- and long-term rates of mortality following acute myocardial infarction. Thus, strict glycemic control may improve outcomes; however, trials of insulin infusion (DIGAMI and DIGAMI 2) were negative in post-MI patients. In other settings, data on insulin infusion and strict glycemic control were more promising. In a trial involving over 1,500 critically ill patients, targeting a blood glucose between 80-110 vs 180-200 mg/dl reduced mortality. The practice appeared to be most beneficial in septic patients.
To resolve uncertainty around intensive glucose control in critically ill patients, which includes patients with AMI, the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial sought to test the hypothesis that intensive glucose control reduces mortality at 90 days.
Patients Patients admitted to the ICUs of 42 hospitals across Australia, Canada and New Zealand with an expected ICU length of stay of at least 3 days. The cause of admission could not be DKA.
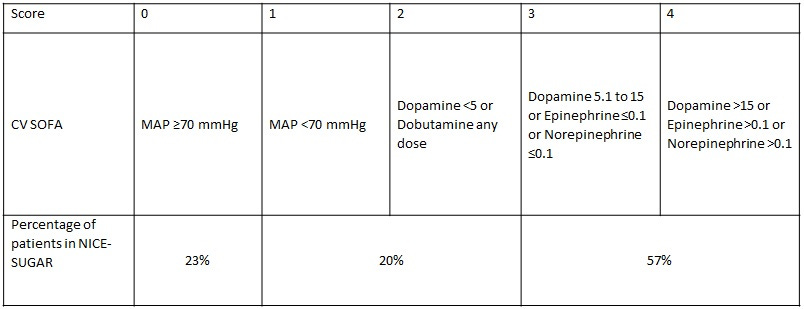
Baseline characteristics There were 40,170 patients who were assessed for eligibility and met inclusion criteria and 6,104 (15%) underwent randomization. The most common reason for exclusion was the expectation that patients would be eating within 24 hr. The average age of participants was 60 years and 62% were men. The indication for ICU admission was nonoperative in 63%. The average blood glucose at the time of randomization was 145 mg/dl. The mean APACHE II score was 21. Most patients had evidence of cardiovascular dysfunction and greater than 50% had a cardiovascular SOFA score of 3-4, indicating severe cardiovascular failure.
Procedures An intravenous infusion of insulin in saline was used to control blood glucose in all patients who were assigned to either strict blood glucose control, which targeted a blood glucose level of 81 to 108 mg/dl or conventional care, which targeted a level of ≤180 mg/dl. Blood glucose levels were managed as part of the normal duties of the clinical staff at the participating center and in both groups, were guided by treatment algorithms accessed through a secure Web site. Blood glucose measurements were obtained by means of arterial catheters whenever possible; the use of capillary samples was discouraged.
The trial intervention was discontinued once the patient was eating or was discharged from the ICU but was resumed if the patient was readmitted to the ICU within 90 days. It was discontinued permanently at the time of death or 90 days after randomization, whichever occurred first.
Endpoints The primary endpoint was all-cause mortality within 90 days. The investigators hypothesized that strict glucose control would reduce the absolute rate of mortality by 3.8%, from 30% in the conventional group to 26.2% in the strict control group. Based on that assumption, a sample size of 6,100 patients was needed to demonstrate the expected mortality reduction with a 5% significance level and power of 90%.
Results 6,140 patients were randomized but consent was withheld or withdrawn for 74 patients leaving a final sample size of 6,066 with 3,016 in the strict control arm and 3,014 in the conventional arm. The time averaged blood glucose level was significantly lower in the strict control arm (115 vs 144 mg/dl) and insulin use was much higher (50 vs 17 units per day).
Strict glucose control did not significantly reduce all-cause mortality at 90 days; instead, it increased it (OR 1.14; 27.5% vs 24.9%; p=0.02). Possible subgroup interactions were noted for trauma patients as well as those on corticosteroids but caution is urged in interpreting these results as they were very small subgroups with wide confidence intervals. Strict glucose control significantly increased severe hypoglycemia with blood glucose <40 mg/dl (6.8% vs 0.5%; p<0.001).
Conclusions Strict blood glucose control increased mortality and severe hypoglycemia compared to conventional control in critically ill patients. The majority of patients had cardiovascular dysfunction and more than half had severe cardiac failure based on SOFA scores.
While this trial was not a trial of AMI patients, we have included it because it is the largest and most comprehensive trial involving intensive blood glucose control and AMI patients would be included in its target population of critically ill patients.
In contemporary practice, glucose targets of 180 mg/dl are commonly employed and advocated for; however, it should be noted that this represents a subtle moving of the goal posts. NICE-SUGAR compared strict to less strict (<180 mg/dl) and less strict did better; however, there is no reason to suspect, based on the accumulated data that an even less strict target or no target at all would fair any worse. In DIGAMI 2, patients treated to a target non-fasting blood glucose <180 mg/dl did numerically worse than those managed without a defined target.
Stress hyperglycemia is an evolved adaptive response that is present across the animal kingdom. We have no evidence in patients hospitalized with AMI that treating blood glucose to any target is better than not treating it. Despite this, there is strong evidence that blood glucose treatment in hospitalized patients increases hypoglycemia, which from an acute standpoint is more dangerous than hyperglycemia.
We urge extreme caution in treating stress hyperglycemia in AMI patients, including those with critical illness.