Revisiting the PLATO trial
A recent BMJ investigation has rekindled the robust debate surrounding the PLATO trial of ticagrelor vs clopidogrel in patients with acute coronary syndromes
Our initial review of the PLATO trial, published in April 2024, was based on the data available to us at that time.
We have since became aware of new information that reduces our confidence in the PLATO results. This new information has major implications for clinical practice.
Despite representing only 6.9% of the total P2Y12 inhibitor prescriptions among Medicare beneficiaries in 2020, Ticagrelor accounted for nearly two-thirds of total Medicare spending on these drugs in the same year.
We summarize important points below but you can refer to this investigation at BMJ for more details.
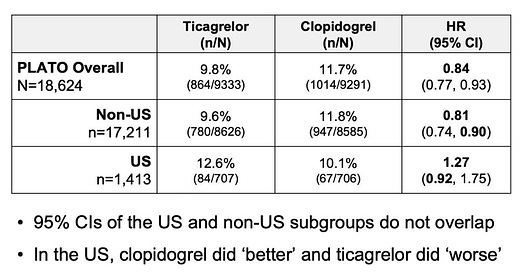
1. Unexplained Regional Variation: In our original review, we highlighted the treatment effect interaction based on region of enrollment, where ticagrelor was less effective compared to clopidogrel for patients enrolled in North America.
It appeared to be a strong signal and was associated with a p-value for the interaction of 0.05. However, we were cautious in our interpretation since overall, patients enrolled in North America represented a relatively small fraction of total patients and we could not think of a reasonable explanation.
Information in the BMJ investigation now sheds new light on these findings. In our review, we only presented data contained in the supplement accompanying the PLATO trial manuscript, which categorized patients based on region of enrollment but did not provide country specific information.
The BMJ report notes that in a separate subgroup analysis, based on country of randomization, the primary outcome was numerically higher with ticagrelor in the United States (12.6% vs 10.1%, HR: 1.27, 95% CI: 0.92 – 1.75). This subgroup represented 7.6% of the total trial participants. Overall, 9.7% of trial participants were enrolled from North America. This means the US data drove the findings from the North American subgroup.
The explanation provided by AstraZeneca (the manufacturer of ticagrelor) to explain the observed treatment effect heterogeneity was that aspirin dosing in the United States was higher than in other countries. It even led the FDA to issue a black box warning to avoid an aspirin maintenance dose of >100 mg in patients taking ticagrelor.
An extensive statistical analysis of the regional variation in PLATO yielded four interesting points. First what was the prior likelihood of observing a ticagrelor vs clopidogrel HR of > 1.25 in the US, when the overall HR was actually equal to 0.84? That probability is ≤ 0.01. This alone suggests more than chance. Second point: a strong US/nonUS interaction was noted for each of the 3 components of the primary endpoint—CV death, MI, stroke. Third: they found a very strong interaction between treatment and median aspirin dose, and, importantly, the aspirin interaction effect was similar in US and nonUS settings. Fourth, an analysis of contract research organization (CRO) vs sponsor monitoring of the site accounted for 61% of the treatment-by- region interaction. The authors downplayed this finding because of the four countries monitored by a CRO (Israel, US, Georgia and Russia), the US made up the majority and thus is confounded by the aspirin interaction. Noteworthy was a lack of direct analysis of CRO vs sponsor test for interaction.
One problem though: the BMJ investigation found that the lead author, Kevin Carroll was the head statistician at AstraZeneca and had worked at the company for 20 years. Carroll presented the PLATO results at the FDA advisory meeting. The paper lists Carroll as having no conflicts. Carroll told the BMJ that he had disposed of all conflicts of interest before submitting that analysis.
But, in our opinion, the aspirin explanation does not pass muster because of biologic implausibility. See next section:
How would a higher dose of aspirin reduce the efficacy of ticagrelor?
The primary composite endpoint was vascular death, MI or stroke. If the higher aspirin dose impacted this, we would hypothesize that it caused more major bleeding in the ticagrelor group with some events resulting in vascular death, type 2 MI and hemorrhagic stroke, driving the treatment effect in favor of clopidogrel. But there is no evidence of this.
The figure below is from the original subgroup plots provided in the PLATO supplement. The difference in the treatment effect for the primary endpoint for North American patients is striking but there is no difference for major bleeding.
In our opinion and the opinion of others, the role of supervision of the centers could be important. Most centers were monitored by the sponsor. Four countries (Israel, US, Georgia and Russia) were monitored by a contract research organization. All four of these countries had numerically higher rates of the primary outcome in the ticagrelor group.
This has major implications and we do not take them lightly. Essentially, it suggests malfeasance on the part of the sponsor. So is there anything else to support such a claim? Well, yes.
2. Concerns about event adjudication. Based on a report from Victor Serebruany, an adjunct faculty member at Johns Hopkins University, and the BMJ investigation, FDA records indicated that site reports documented 504 myocardial infarctions in patients who received ticagrelor compared to 548 in patients who received clopidogrel. However, after adjudication, the count increased only for the clopidogrel group, reaching 593. There was also some imbalance among groups in adjudicating death. These imbalances raise concerns about potential unblinding and result tampering. We read many of the authors’ replies and we did not find a clear explanation of why all readjudicated extra MIs were in the clopidogrel group (45 clopidogrel; ticagrelor 0).
3. There were also concerns about the accuracy of death records as sites death records did not always match the FDA records.
We cite from the BMJ:
The BMJ’s analysis also found omissions in PLATO’s landmark publication. The paper, published in NEJM and reported as an intent-to-treat analysis, reports 905 total deaths from any cause among all randomized patients. An internal company report states, however, that 983 patients had died at this point. While 33 deaths occurred after the follow-up period, the NEJM tally still leaves out 45 deaths “discovered after withdrawal of consent.” The BMJ obtained some records for patients whose deaths were not reported in NEJM (see table 1) and asked the journal for a response.
NEJM editor in chief Eric Rubin told The BMJ that “for older manuscripts, correction is not necessarily appropriate unless there would be an effect on clinical practice,” concluding that “it does not appear that correcting this 15-year-old article is going to have any impact.”
It is noteworthy that the United States Department of Justice launched a formal investigation into the PLATO trial in 2013; however, the probe was closed in 2014. The BMJ column cited a spokesperson for the US attorney’s office who said…”we determined that the allegations lacked sufficient merit such that it was not in the best interests of the US to intervene in the suit.”
4. Mortality reduction in PLATO defies explanation: Shortly after PLATO was published, Drs. Victor Serebruany and Dan Atar wrote an editorial in the European Heart Journal titled: The PLATO trial: do you believe in magic?
They noted that the overall HR for all-cause death ticagrelor vs clopidogrel was 0.78 (95% CI: 0.69 - 0.89; p< 0.001). There were 107 more lives saved with ticagrelor vs clopidogrel. To explain the surprise of this massive effect size, they compared it to the COMMIT trial of clopidogrel vs placebo in patients with acute MI. In COMMIT, 119 lives were saved with clopidogrel (vs placebo), but COMMIT had three-fold more patients than PLATO—and the gain was vs placebo. They tempt the reader to ask: how could ticagrelor fare that well against a drug that crushed placebo?
We note two other reasons to be concerned about the outsized mortality reduction in PLATO. One is plausibility. The all-cause mortality benefit exceeded the reduction in MI, CV death or stroke. Given the numerically higher rate of bleeding, how else does ticagrelor reduce death vs clopidogrel? The second reason is the lack of such a signal in Phase 2 studies, such as this one.
5. PLATO results are on outlier: Multiple observational studies have failed to replicate the benefits of ticagrelor observed in the PLATO trial. While observational studies are inherently limited by confounding factors and are inferior to randomized trials, their findings warrant a re-evaluation of ticagrelor’s benefits. Furthermore, two randomized trials—one conducted predominantly in Japanese patients and another in South Korea—did not demonstrate the superiority of ticagrelor, instead showing higher bleeding rates and a numerical increase in ischemic events.
Ticagrelor also significantly underperformed against another new antiplatelet drug, prasugrel. In the non-industry-funded ISAR-REACT 5 trial, which enrolled patients with acute coronary syndrome, the primary event of death, MI, or stroke was 36% higher in the ticagrelor arm (9.3% vs 6.9%, HR 1.36, 95% CI: 1.09 - 1.70). Major bleeding was also numerically higher in the ticagrelor arm.
6. PLATO authors have responded to these arguments.
We provide links to four of the authors responses.
Thrombosis and Hemostasis https://www.wellesu.com/10.1160/TH11-03-0162
Stroke https://www.ahajournals.org/doi/10.1161/strokeaha.111.000514
Inter J of Cardiol https://doi.org/10.1016/j.ijcard.2014.06.029
Circulation https://doi.org/10.1161/CIRCULATIONAHA.111.047498
Conclusion
These are vitally important revelations regarding PLATO and ticagrelor. The FDA advisory committee recommended that FDA require a confirmatory trial. This was not done. As such, ticagrelor gained serious market share in the non-clopidogrel antiplatelet market for more than a decade.
Yet no other compelling evidence for its benefit over clopidogrel has come to light. It clearly underperformed vs prasugrel.
These old and new revelations have changed our positive view of ticagrelor. We no longer have confidence in this drug.
We strongly agree with the recommendation for another properly controlled trial. We also believe this highlights the benefits of having either two regulatory trials or a single regulatory trial combined with a mandated post-approval trial.
These revelations also emphasize the benefits of robust critical appraisal and skeptical but not cynical approaches to surprising evidence.